18-Dec-2020 | Facts and Factors
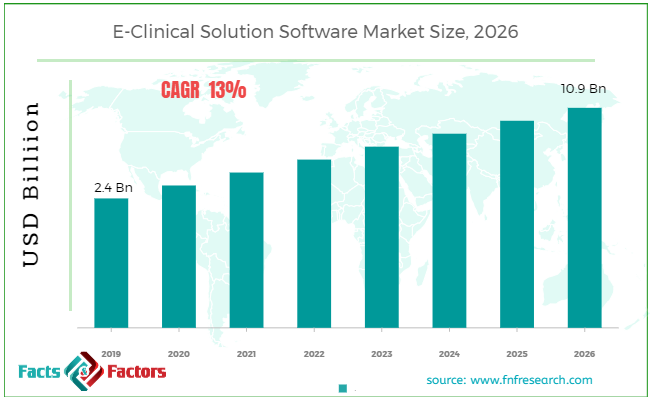
Findings from Facts and Factors report states that the global E-Clinical Solution Software Market in 2019 was approximately USD 2.4 billion. The market is expected to grow at a CAGR of 13% and is anticipated to reach around USD 10.9 billion by 2026.
The Global E-Clinical Solution Software Market Is Powered By Increase in clinical trials and higher investments in R&D activities in the fields of life science and pharmaceuticals.
The type of software which helps the researchers in tracking, analyzing o managing the data related to clinical is termed e-clinical solution software. This software is widely used by biotechnology, pharmaceutical companies, research organizations for medical device designing to coagulate the data for clinical research by the use of modern cutting edge technology. There is a need for clinical trials for judging the safety and efficiency of drugs, medical devices, and therapeutic products before their launch in the market.
The high growth of the internet globally and the easy access availability will propel the market growth. Additionally, higher efficacy, as well as credible data analysis as compared to the conventional process, will enhance the players to adopt this e-clinical solution software for clinical-based trials.
Browse the full “E-Clinical Solution Software Market By Product (Electronic Trail Master Files, Electronic Data Capture and Clinical Data Management Systems, Clinical Outcome Assessment, Clinical Analytics Platform, Randomization, and Trial Supply Management, Clinical Data Integration Platform, Regulatory Information Management Solutions, Safety Solutions, and Others), By Delivery Mode (Licensed Enterprise, Web Hosted, and Cloud-Based), By Clinical Trials (Phase I, Phase II, Phase III, and Phase IV), By End-User (Contract Research Organizations, Medical Device Manufacturers, Academic Research Institutions, Pharmaceutical and Biopharmaceutical Companies, Consulting Service Companies, and Hospitals): Global Industry Outlook, Market Size, Business Intelligence, Consumer Preferences, Statistical Surveys, Comprehensive Analysis, Historical Developments, Current Trends, and Forecasts, 2020–2026" report at https://www.fnfresearch.com/e-clinical-solution-software-market
The high rise in clinical trials, as well as research and development in life sciences and pharmaceutical sectors, is growing the market of e-clinical solution softwares globally. Also, there is a rise in diseases that supports clinical trials in various regions. Lifestyle-related diseases, as well as genetic disorders in North African and the Middle East countries, have also enhanced the clinical trials. Moreover, the pharmaceutical industry is witnessing high pressure to improve the productivity of drugs and to also reduce the time required for clinical trials. E-clinical software is accepted by industry verticals such as pharmaceutical, clinical research organizations among others.
The high demand for the production of new drugs by the use of clinical trials and research and development activities is expected to boost the demand for the e-clinical solution software market globally. The market is also growing because of the increase in the need for efficient data management and also standardization of the data. There are numerous benefits that are associated with e-clinical solution softwares such as reduction of time in clinical trials, improvement in the productivity of drugs due to which the end-use industries will adopt this latest technology. The topmost end-use industries in the e-clinical solution softwares are pharmaceutical companies, the healthcare sector, clinical research organizations. Among these end-use industries, the pharmaceutical sector will be of high market share because most of the companies are focusing on the innovation of a new line of drugs.
The market for e-clinical solution software is segmented based on delivery mode, product, clinical trial phase, end-user, and region. Based on product the market is bifurcated into electronic trial master files, electronic data capture and clinical data management systems, clinical outcome assessment, clinical analytics platform, randomization, and trial supply management, clinical data integration platform, regulatory information management solutions, safety solutions, and others.
On the basis of delivery mode, the market is segregated into the licensed enterprise, web-hosted, and cloud-based. Further in terms of clinical trials, the market is categorized into phase I, phase II phase III, phase IV. In terms of geography, North America will dominate the market due to the rise in preference for advancement in technology by organizations and institutes. Also, the increase in investments by the government, the number of clinical trials, and the growth of pharmaceutical companies are some of the major factors due to which there is the huge rise in the North American region.
Report Scope
Report Attribute |
Details |
Market Size in 2019 |
USD 2.4 Billion |
Projected Market Size in 2026 |
USD 10.9 Billion |
CAGR Growth Rate |
13% CAGR |
Base Year |
2019 |
Forecast Years |
2020-2026 |
Key Market Players |
Medidata Solutions, Inc, Merge Healthcare Incorporated, BioClinica, Inc., Bio-Optronics, Inc., DATATRAK International, ERT, MaxisIT Inc., CRF Health, eClinical Solutions, Inc., PAREXEL International Corporation, OmniComm Systems, Inc., and Others |
Key Segment |
By Application, and Region |
Major Regions Covered |
North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
Purchase Options |
Request customized purchase options to meet your research needs. Explore purchase options |
Some of the leading and top companies in the e-clinical solution software market include
Medidata Solutions, Inc, Merge Healthcare Incorporated, BioClinica, Inc., Bio-Optronics, Inc., DATATRAK International, ERT, MaxisIT Inc., CRF Health, eClinical Solutions, Inc., PAREXEL International Corporation, OmniComm Systems, Inc., among others.
The taxonomy of the e-clinical solution software market by its scope and segmentation is as follows:
By Product Analysis
- Electronic Trial Master Files
- Electronic Data Capture and Clinical Data Management Systems
- Clinical Outcome Assessment
- Clinical Analytics Platform
- Randomization and Trial Supply Management
- Clinical Data Integration Platform
- Regulatory Information Management Solutions
- Safety Solutions
- Others
By Delivery Mode Analysis
- Licensed Enterprise
- Web Hosted
- Cloud-Based
By Clinical Trials Analysis
- Phase I
- Phase II
- Phase III
- Phase IV
By End-UserAnalysis
- Contract Research Organizations
- Medical Device Manufacturers
- Academic Research Institutions
- Pharmaceutical and Biopharmaceutical Companies
- Consulting Service Companies
- Hospitals
By Regional Segmentation Analysis
- North America
- Europe
- Germany
- The UK
- France
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
About Us:
Facts & Factors is a leading market research organization offering industry expertise and scrupulous consulting services to clients for their business development. The reports and services offered by Facts and Factors are used by prestigious academic institutions, start-ups, and companies globally to measure and understand the changing international and regional business backgrounds. Our client’s/customer’s conviction on our solutions and services has pushed us in delivering always the best. Our advanced research solutions have helped them in appropriate decision-making and guidance for strategies to expand their business.
Contact Us:
Facts & Factors
A 2108, Sargam,
Nanded City,
Sinhagad Road,
Pune 411041, India
USA: +1 (347) 690-0211
Email: [email protected]
Web: https://www.fnfresearch.com