09-Jul-2024 | Facts and Factors
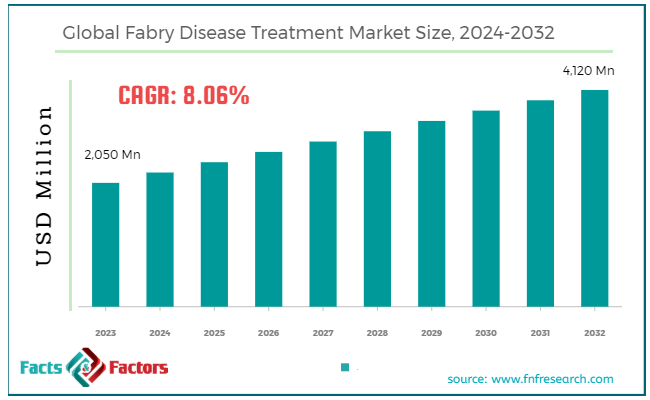
As per the market research report published by Facts and Factors, the global Fabry disease treatment market was valued at approximately USD 2050 million in 2023 and is expected to generate revenue of around USD 4,120 million by end of 2032, growing at a CAGR of around 8.06% between 2024 and 2032.
Fabry disease is a very rare disease. The deficiency of the alpha-galactosidase enzyme in a patient causes progressive organ dysfunction. The development of Fabry diseases is primarily caused by an abnormal accumulation of a particular fatty matter known as globotriaosylceramide. The skin, heart, eyes, brain, kidneys, central nervous system, and gastrointestinal system are all affected by this irregular accumulation.
Browse the full “Fabry Disease Treatment Market Size, Share, Growth Analysis Report By Treatment (Substrate Reduction Therapy (SRT), Enzyme Replacement Therapy (ERT), Pharmacological Chaperone Therapy, and Others), By Route of Administration (Oral and Intravenous), By End-User (Hospitals, Specialty Clinics, and Research Institutions), and By Region - Global Industry Insights, Overview, Comprehensive Analysis, Trends, Statistical Research, Market Intelligence, Historical Data and Forecast 2024 – 2032” report at https://www.fnfresearch.com/fabry-disease-treatment-market-report.
Owing to the presence of only one X chromosome in the male, they are more prone to such disorder. On the other hand, the population of females acts as carriers and is less likely to experience disease complications in the early stages. However, neurological problems such as high blood pressure, heart failure, stroke, and other complications have been known to evolve. The main symptoms are burning sensations, excessive sweating, cloudy vision, irregular bowel movements, and other symptoms that are the most common in this disorder. It is identified by testing the -gal enzyme in the patients' leukocytes. In the case of neonates, the presence of this disease is identified when assessed for improper heart or kidney development. Since there is no cure for Fabry disease, treatment focuses on preventing complications from developing and providing symptomatic relief. Mild symptoms often lead to misdiagnosis in a variety of patients. Certain factors are anticipated to expand the market at a significant rate in the next few years.
The Existence Of A Strong Drug Pipeline For The Treatment Of Fabry Disease Is Expected To Drive Development.
Several regulatory bodies in key regions have recently approved novel drugs to treat Fabry disease. Citing an instance, in 2018, Galafold, a medication developed by Amicus Therapeutics, Inc., has been approved by the US Food and Drug Administration (FDA) (migalastat). It is the first oral drug approved for the treatment of Fabry disease specifically for adults. Furthermore, market players are massively investing in the production of novel therapies for the treatment of Fabry disease and strategic expenditure by various players will proliferate the global market growth in upcoming years. For instance, AvroBio Inc. announced the completion of a $60 million funding round to fund the phase 2 trial of AVR-RD-01, novel gene therapy in development for the treatment of Fabry disease in February 2018. The market's growth will be fueled by these factors over the forecast period.
On the other hand, Fabry disease affects one among fifty thousand people as stated by National Fabry Disease Foundation. Moreover, the diagnosis of Fabry disease is verified by illustrating deficiency of an enzyme among the male population and finding the unique GLA gene mutation in females and males. As a result, low diagnostic rates in emerging economies due to a lack of such diagnostic procedures and qualified professionals are anticipated to hinder the growth of the global Fabry disease treatment market.
COVID-19 Impact on the Global Fabry Disease Treatment Market
COVID-19's effect on Fabry disease patients undergoing enzyme replacement therapy is still unknown. Fear of infection caused several patients receiving care in the hospital to have their infusions interrupted. In other lysosomal storage diseases, the effects of temporary treatment interruption have been identified in greater detail, but resuming therapy does not completely reverse clinical deterioration caused by the temporary interruption. Home therapy seems to be the most effective way to sustain enzyme replacement therapy access during a pandemic when it is necessary. During the forecast period, these factors are expected to propel the market growth.
North America is projected to Dominate Global Fabry Disease Treatment Market Growth
In 2019, the global Fabry disease treatment market was dominated by the North American region, which accounted for the largest market share. Higher acceptance of new therapies, favorable reimbursement policies, and improved healthcare services are all contributing to the region's market development. Drug companies are increasing their R&D investments in the field of rare diseases as a result of health insurance schemes covering health expenditure costs.
Due to various improved healthcare infrastructure and increasing healthcare spending in emerging economies, Asia Pacific offered a huge growth opportunity for pharmaceutical companies. This region is expected to hold a larger share of the market during the forecast period. Furthermore, the availability of biosimilars is expected to make these therapies more affordable in this field. In 2019, as stated by the Ministry of Health and Family Welfare, in India, the average annual cost of enzyme replacement therapy was USD 26,334. These factors are considered to propel the demand in the region in upcoming years.
Report Scope
Report Attribute |
Details |
Market Size in 2023 |
USD 2,050 Million |
Projected Market Size in 2032 |
USD 4,120 Million |
CAGR Growth Rate |
8.06% CAGR |
Base Year |
2023 |
Forecast Years |
2024-2032 |
Key Market Players |
Amicus Therapeutics, Avrobio Inc., Chiesi Group, Green Cross Corporation, Idorsia Pharmaceuticals, JCR Pharmaceuticals, Moderna Therapeutics Inc., Protalix BioTherapeutics, Regenxbio Inc., Sanofi Genzyme, Shire Plc., and Others. |
Key Segment |
By Treatment, By Route of Administration, By End-User, and By Region |
Major Regions Covered |
North America, Europe, Asia Pacific, Latin America, and the Middle East &, Africa |
Purchase Options |
Request customized purchase options to meet your research needs. Explore purchase options |
Top Market Players
Major players operative in the global Fabry disease treatment market are Amicus Therapeutics, Avrobio Inc., Chiesi Group, Green Cross Corporation, Idorsia Pharmaceuticals, JCR Pharmaceuticals, Moderna Therapeutics Inc., Protalix BioTherapeutics, Regenxbio Inc., Sanofi Genzyme, Shire Plc., and others.
The global Fabry disease treatment market is segmented as follows:
By Treatment
- Substrate Reduction Therapy (SRT)
- Enzyme Replacement Therapy (ERT)
- Pharmacological Chaperone Therapy
- Others
By Route of Administration
By End-User
- Hospitals
- Specialty Clinics
By Region:
- North America
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of Middle East & Africa
About Us:
Facts & Factors is a leading market research organization offering industry expertise and scrupulous consulting services to clients for their business development. The reports and services offered by Facts and Factors are used by prestigious academic institutions, start-ups, and companies globally to measure and understand the changing international and regional business backgrounds. Our client’s/customer’s conviction on our solutions and services has pushed us in delivering always the best. Our advanced research solutions have helped them in appropriate decision-making and guidance for strategies to expand their business.
Contact Us:
Facts & Factors
A 2108, Sargam,
Nanded City,
Sinhagad Road,
Pune 411041, India
USA: +1 (347) 690-0211
Email: [email protected]
Web: https://www.fnfresearch.com