26-Mar-2020 | Facts and Factors
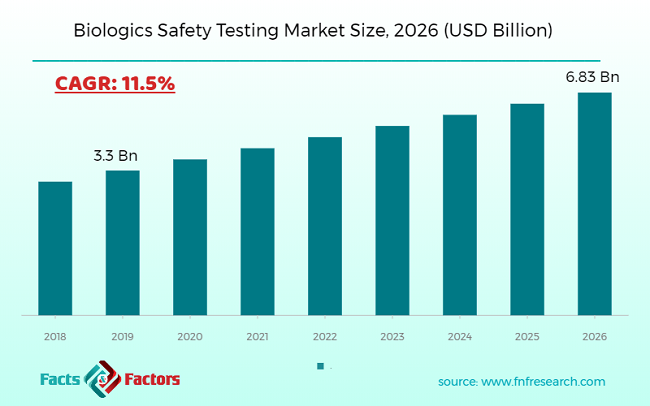
The global biologics safety testing market in 2019 was approximately USD 3.3 Billion. The market is expected to grow above a CAGR of 11.5% and is anticipated to reach over USD 6.83 Billion by 2026.
Biologics products are applied to products from biological sources that are produced. Biologics are used as medical items in animals and humans. Such biologics contain serum and blood products, large polypeptides, vaccines, antitoxins, toxins, viruses, etc. The protection of biologics is an important criterion for its use in animals and humans and also for regulatory approvals. The demand for the biologics safety testing market is driven by growing demand for biologics to address the burden of various chronic diseases, rising investment in life science research and development, rapid growth in new product launches, growth in pharmaceutical and biopharmaceutical research, and increased prevalence of chronic disorders.
Browse the full “Biologics Safety Testing Market By Product (Services, Instruments and Reagents, and Kits); by Test Type (Bioburden tests, Adventitious Agent Detection Tests, Residual Host Contamination Detection Tests, Cell Line Authentication and Characterization Tests, Sterility Tests, Endotoxin Tests, and Other Test Types); by Application (Tissue and Tissue-related Products Testing, Cellular and Gene Therapy, Blood and Blood-related Products Testing, Vaccine and Therapeutics Development and Stem Cell Research): Global Industry Outlook, Market Size, Business Intelligence, Consumer Preferences, Statistical Surveys, Comprehensive Analysis, Historical Developments, Current Trends, and Forecasts, 2020–2026" https://www.fnfresearch.com/biologics-safety-testing-market-by-product-services-instruments-946
The global biologics safety testing market has been segmented on the basis of product, test type, and application. On the basis of product segment, the target market is segmented into reagents and kits, services, and instruments. Also, on the basis of test type segment the global market is segmented into endotoxin tests, sterility tests, cell line authentication and characterization tests, residual host contamination detection tests, adventitious agent detection tests, bioburden tests, and other tests. The application segment is bifurcated as vaccine and therapeutics development, blood and blood-related product testing, cellular and gene therapy, tissue and tissue-related product testing, and stem cell research.
North America continues to dominate the global market for the target market, owing to the presence of key operating players in the target market. Also, the availability of advanced healthcare facilities in the countries if this region is also propelling the growth of the target market in this region. Furthermore, growth in the focus on the new vaccine and therapeutics development by pharmaceutical and biopharmaceutical companies is expected to fuel market growth in the region. The Asia Pacific market is expected to rise at a faster rate of growth as a result of increased awareness among people, increased investment in R&D, the use of skilled labor to produce products, and increased investment by the pharmaceutical industry. Also, the market in the Middle East and Africa is expected to show lucrative growth opportunities in the countries of this region.
Report Scope
Report Attribute |
Details |
Market Size in 2019 |
USD 3.3 Billion |
Projected Market Size in 2026 |
USD 6.83 Billion |
CAGR Growth Rate |
11.5% CAGR |
Base Year |
2020 |
Forecast Years |
2020-2026 |
Key Market Players |
Merck KGaA, Pace Analytical Services Inc., Charles River Laboratories, Toxikon Corporation, Cytovance Biologics, Inc., Sartorius AG, Thermo Fisher Scientific Inc., WuXi Apptec, SGS SA, Lonza Group LTD. and among others. |
Key Segment |
By Product, By Test Type, By Application, By Region |
Major Regions Covered |
North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
Purchase Options |
Request customized purchase options to meet your research needs. Explore purchase options |
Top key players operating in the market are Merck KGaA, Pace Analytical Services Inc., Charles River Laboratories, Toxikon Corporation, Cytovance Biologics, Inc., Sartorius AG, Thermo Fisher Scientific Inc., WuXi Apptec, SGS SA, Lonza Group LTD. and among others. The key players operating in the global biologics safety testing market are focusing on organic as well as inorganic growth strategies to get a competitive edge in the global market. Innovations and the launch of advanced products are creating opportunities in the target market.
This report segments the Biologics Safety Testing market as follows:
Global Biologics Safety Testing Market: By Product
- Reagents and Kits
- Services
- Instruments
Global Biologics Safety Testing Market: By Test Type
- Endotoxin Tests
- Sterility Tests
- Cell Line Authentication and Characterization Tests
- Residual Host Contamination Detection Tests
- Adventitious Agent Detection Tests
- Bioburden tests
- Other Tests
Global Biologics Safety Testing Market: By Application
- Vaccine and Therapeutics Development
- Blood and Blood-related Products Testing
- Cellular and Gene Therapy
- Tissue and Tissue-related Products Testing
- Stem Cell Research
Global Biologics Safety Testing Market Market: Regional Segmentation Analysis
- North America
- Europe
- Germany
- The UK
- France
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of the Middle East & Africa
About Us:
Facts & Factors is a leading market research organization offering industry expertise and scrupulous consulting services to clients for their business development. The reports and services offered by Facts and Factors are used by prestigious academic institutions, start-ups, and companies globally to measure and understand the changing international and regional business backgrounds. Our client’s/customer’s conviction on our solutions and services has pushed us in delivering always the best. Our advanced research solutions have helped them in appropriate decision-making and guidance for strategies to expand their business.
Contact Us:
Facts & Factors
A - 2108, Sargam,
Nanded City,
Sinhagad Road,
Pune 411041, India
USA: +1-347-989-3985
Email: [email protected]
Web: https://www.fnfresearch.com