Search Market Research Report
Clinical Trials Market Size, Share Global Analysis Report, 2020 – 2026
Clinical Trials Market By Phase (Phase I, Phase II, Phase III, and Phase IV), by Indication (Autoimmune/Inflammation, Pain management, Oncology, CNS condition, Diabetes, Obesity, Cardiovascular, and Others): Global Industry Perspective, Comprehensive Analysis and Forecast 2020 – 2026
Industry Insights
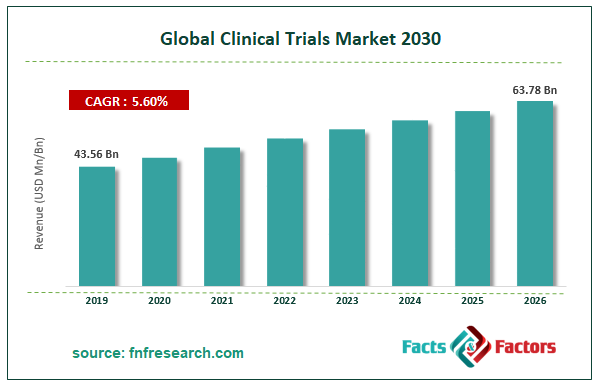
[195+ Pages Report] According to the report published by Facts and Factors, the global clinical trials market was valued at approximately USD 43.56 Billion in 2019 and is expected to generate revenue of around USD 63.78 Billion by end of 2026, growing at a CAGR of around 5.6% between 2020 and 2026.
 Market Overview
Market Overview
A clinical trial is a clinical study that involves administering a test or treatment to a group of people. Clinical studies look at the effectiveness and safety of experiments and therapies. The test or procedure is approved as a standard of care if it is effective and meets regulatory criteria. Because of the globalization of clinical trials, the emergence of innovative treatments such as personalized medicine, and the prevalence of infection and new disease cases, the clinical trial market is expected to expand in the coming years. Furthermore, growing investments in the development of new healthcare products are expected to provide substantial market growth opportunities over the forecast period.
 Industry Growth Factors
Industry Growth Factors
The high demand for clinical trials in emerging markets, the pharmaceutical industry's high R&D spending, the rising prevalence of diseases, and the emphasis on rare diseases and numerous orphan drugs in the pipeline are all major factors driving the market's growth. Aging is also discovered to be a major factor driving market growth in terms of disease burden and demand for the market studied. With the growing burden of diseases, market players are increasingly focusing on developing new therapeutics for rare or genetic disorders that need expertise and concentrated clinical trials, which is expected to fuel demand for market development. Government programs have also encouraged small, mid, and large companies to join the drug production market. As a result, there are a number of medications in the works.
 Segmentation Analysis
Segmentation Analysis
Phase III is expected to dominate the market, with Phase I expected to rise at the fastest pace. Phase III is one of the most important stages in determining whether or not a new intervention is successful and useful in clinical practice. These phase III clinical trials are used to compare the effects of a new drug to those of previously available drugs, or to validate and build on the safety and efficacy findings from Phase 1 and 2 trials. Because of their greater complexity and need for a wider patient pool, these clinical trials continue to have a higher demand for outsourcing services than phase II and phase I trials. The demand is also expected to expand as a result of government policies in developing economies to promote drug development and continuous technological advances.
 Regional Analysis
Regional Analysis
Because of the involvement of large outsourcing firms and increased R&D in the region, North America has dominated the overall market. This is primarily due to factors such as rising R&D investments and rising demand for drug production, which are the primary drivers of market growth in the United States.
Asia is one of the most rapidly expanding markets for clinical trials worldwide. India, China, South Korea, and Singapore are the leading countries in this industry. The fact that this market has such a large and diverse population of diseases that exist in both developed and developing countries is one of the main reasons for its high potential. As a result, various types of trials can be carried out in the same region. Due to the large population, it facilitates quicker patient recruitment and higher retention of study participants, allowing organizations to complete trials on time.
 Report Scope
Report Scope
Report Attribute |
Details |
Market Size in 2019 |
USD 43.56 Billion |
Projected Market Size in 2026 |
USD 63.78 Billion |
CAGR Growth Rate |
5.6% CAGR |
Base Year |
2019 |
Forecast Years |
2020-2026 |
Key Market Players |
Clinipace, Eli Lilly and Company, ICON Plc, Novo Nordisk A/S, Pfizer, Wuxi AppTec, Syneos Health, SGS SA, PRA Health Sciences, and others. |
Key Segment |
By Phase, By Indication, and By Region |
Major Regions Covered |
North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
Purchase Options |
Request customized purchase options to meet your research needs. Explore purchase options |
 Competitive Players
Competitive Players
- Clinipace
- Eli Lilly and Company
- ICON Plc
- Novo Nordisk A/S
- Pfizer
- Wuxi AppTec
- Syneos Health
- SGS SA
- PRA Health Sciences
- others.
 Clinical Trials Market: Regional Segment Analysis
Clinical Trials Market: Regional Segment Analysis
- North America
- U.S.
- Canada
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East and Africa
- GCC Countries
- South Africa
- Rest of MEA
Industry Major Market Players
- Clinipace
- Eli Lilly and Company
- ICON Plc
- Novo Nordisk A/S
- Pfizer
- Wuxi AppTec
- Syneos Health
- SGS SA
- PRA Health Sciences
Frequently Asked Questions

Copyright © 2024 - 2025, All Rights Reserved, Facts and Factors


