Search Market Research Report
Contract Research Organization (CRO) Services Market Size, Share Global Analysis Report, 2024 – 2032
Contract Research Organization (CRO) Services Market Size, Share, Growth Analysis Report By Service Type (Clinical Trial Services, Preclinical Services, Regulatory Services, Laboratory Services, and Others), By Application (Oncology, Central Nervous System (CNS) Disorder, Cardiovascular Diseases, Infectious Disease, and Others), By End-User (Pharmaceutical And Biotechnology Companies, Medical Device Companies, and Academic Institutes), And By Region - Global and Regional Industry Insights, Overview, Comprehensive Analysis, Trends, Statistical Research, Market Intelligence, Historical Data and Forecast 2024 – 2032
Industry Insights
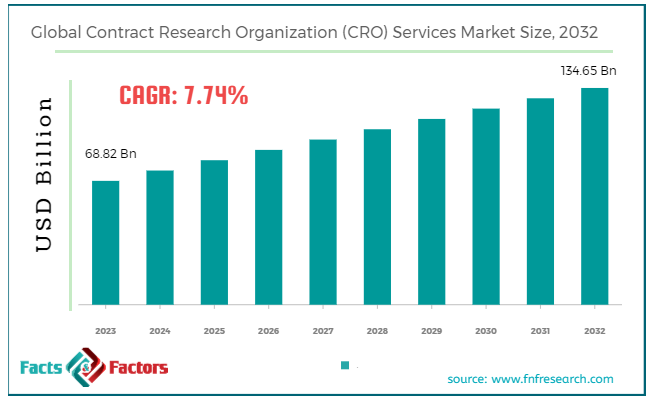
[218+ Pages Report] According to Facts & Factors, the global contract research organization (CRO) services market size in terms of revenue was valued at around USD 68.82 billion in 2023 and is expected to reach a value of USD 134.65 billion by 2032, growing at a CAGR of roughly 7.74% from 2024 to 2032. The global contract research organization (CRO) services market is projected to grow at a significant growth rate due to several driving factors.
 Market Overview
Market Overview
A Contract Research Organization (CRO) provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. CROs offer a comprehensive range of services including drug discovery, clinical trial management, regulatory compliance, and data management, among others. By partnering with CROs, companies can reduce costs, increase efficiencies, and benefit from specialized expertise that accelerates the pace of their drug development processes.
The CRO market has experienced significant growth over the past decade, driven by the increasing complexity of drug development and the need for more efficient and cost-effective research processes. The global pharmaceutical industry's pressure to bring drugs and medical products to market more rapidly has led to the expansion of the CRO sector, as CROs can often complete tasks more efficiently than companies that handle all operations in-house.
 Key Highlights
Key Highlights
- The contract research organization (CRO) services market has registered a CAGR of 7.74% during the forecast period.
- In terms of revenue, the global contract research organization (CRO) services market was estimated at roughly USD 68.82 billion in 2023 and is predicted to attain a value of USD 134.65 billion by 2032.
- The growth of the contract research organization (CRO) services market is being propelled by the expanding scope of research in the pharmaceutical sector, the shift towards personalized medicine, and the increasing complexity of clinical trials. This makes CROs invaluable partners in the quest to bring new therapies and medical devices to market.
- On the basis of Application, Oncology holds the dominant position due to the significant investment in developing new cancer therapies and the complex nature of oncology trials.
- Based on the End Users, the pharmaceutical and biotechnology companies segment is growing at a high rate and is projected to dominate the global market.
- By region, North America is currently the dominating region in the global CRO market, attributed to its advanced healthcare infrastructure, substantial R&D activities, and strong regulatory frameworks.
 Growth Drivers:
Growth Drivers:
- Rising Drug Development Complexity: Developing new drugs is a labyrinthine process demanding specialized knowledge and extensive resources. CRO services provide that expertise, allowing pharmaceutical and biotech companies to leverage their capabilities and navigate the intricacies of drug development.
- Focus on Cost-Effectiveness and Speed: Clinical trials, a major bottleneck in drug development, are streamlined by CRO services. This translates to faster time to market for new treatments and reduced costs for pharmaceutical companies.
- Globalization of Clinical Research: CROs have the experience and infrastructure to manage clinical trials across borders, a crucial aspect in today's world. Global collaboration is essential for efficient drug development, and CRO services facilitate this by navigating international regulations.
 Restraints:
Restraints:
- Intense Competition: The CRO services market is becoming increasingly crowded, with established giants and niche players vying for market share. This fierce competition can lead to price pressures and reduced profit margins for CROs.
- Regulatory Labyrinth: CROs must navigate a complex web of regulations that vary significantly by country. Non-compliance with these regulations can lead to delays, fines, and reputational damage for CROs.
- Data Security Concerns: CRO services handle a vast amount of sensitive patient data. Ensuring robust data security measures are in place is critical, but data breaches can be a major concern.
 Opportunities:
Opportunities:
- Technological Advancements: Emerging technologies like big data analytics, artificial intelligence (AI), and telemedicine hold immense potential for CRO services. These advancements can further streamline trials, improve data analysis, and enhance overall efficiency.
- Rise of Personalized Medicine: The growing focus on personalized medicine creates opportunities for CROs to develop specialized expertise in conducting targeted clinical trials for niche patient populations. This allows for more effective drug development.
- Increased Demand from Emerging Markets: Developing economies are witnessing a surge in healthcare spending and clinical trial activity. This presents a significant growth opportunity for CRO service providers with a global presence.
 Challenges:
Challenges:
- Talent Acquisition and Retention: The CRO services industry requires highly skilled professionals with expertise in clinical research, regulatory affairs, and data management. Finding and retaining such talent can be challenging for CROs.
- Maintaining Quality Standards: As CROs manage an increasing volume of trials, ensuring consistent quality across all projects becomes crucial. Maintaining rigorous quality control measures is an ongoing challenge for CRO service providers.
- Evolving Regulatory Landscape: Regulations governing clinical trials are constantly evolving. CROs need to stay updated on these changes to ensure compliance and avoid disruptions in their services.
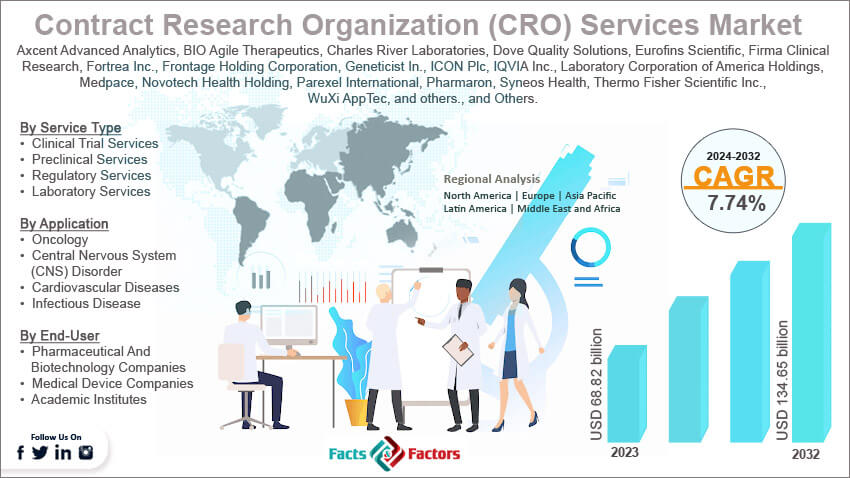
 Contract Research Organization (CRO) Services Market: Segmentation Analysis
Contract Research Organization (CRO) Services Market: Segmentation Analysis
The global contract research organization (CRO) services market is segmented based on service type, application, end-user, and region.
 By Service Type Insights
By Service Type Insights
Based on Service Type, the global contract research organization (CRO) services market is bifurcated into clinical trial services, preclinical services, regulatory services, laboratory services, and others. The clinical trial services segment is the largest and most crucial within the CRO market. It encompasses all phases of clinical trials from Phase I to Phase IV. Clinical trial services are fundamental to the drug development process, offering comprehensive management, patient recruitment, monitoring, and data handling. This segment benefits from the increasing number of drug development programs and the globalization of clinical trials. It typically shows a high CAGR due to the rising demand for outsourcing these complex activities.
Preclinical services involve early-stage research, including pharmacokinetics, toxicology studies, and first-in-human studies. These services are critical for determining a drug candidate's safety profile before it enters clinical trials. While smaller in revenue compared to clinical trials, this segment is essential for early drug development stages. Regulatory services provide support in navigating the complex regulatory landscapes across different countries. This includes regulatory submission, compliance, and consulting services, which are crucial for gaining approval for new drugs and continuing market access.
 By Application Insights
By Application Insights
On the basis of Application, the global contract research organization (CRO) services market is categorized into oncology, central nervous system (CNS) disorders, cardiovascular diseases, infectious diseases, and others. Oncology is the leading therapeutic area within the CRO market, driven by the high number of cancer research projects and the increasing global cancer burden. The complexity of cancer treatments often requires specialized clinical trials, making oncology a high-growth segment with a significant CAGR.
The central nervous system (CNS) disorder segment deals with diseases of the central nervous system and requires specific expertise due to the complexity of disorders like Alzheimer's, Parkinson's, and multiple sclerosis. While it commands a smaller market share than oncology, its growth is spurred by the urgent need for new therapies in neurology. Given the global prevalence of cardiovascular diseases, this area remains a key focus for pharmaceutical research and development. CROs offering specialized services in this therapeutic area help address the large-scale demand for cardiovascular clinical trials.
 By End-User Insights
By End-User Insights
Based on End-User, the global contract research organization (CRO) services market is categorized into pharmaceutical and biotechnology companies, medical device companies, and academic institutes. The pharmaceutical and biotechnology companies segment accounted for 50.2% of revenue share in 2023. The pharmaceutical and biotechnology companies are the predominant end-user segment, as these companies are the primary clients of CROs, outsourcing a significant portion of their research activities to reduce costs and leverage specialized expertise.
Medical device companies are smaller in comparison, this segment is growing due to the increasing complexity of medical device regulations and the specialized nature of medical device clinical trials.
 Recent Developments:
Recent Developments:
- Curavit Launches HEOR Practice for Digital Therapies (Oct 2023): Virtual CRO Curavit expands its offerings by launching a dedicated Health Economics and Outcomes Research (HEOR) practice. This new service focuses on digital therapies (DTx) within decentralized clinical trials (DCTs). By integrating HEOR into trials, Curavit aims to demonstrate the economic value of DTx interventions, potentially accelerating their market adoption.
- IQVIA and argenx Collaborate on Autoimmune Disease Treatments (Oct 2023): IQVIA and argenx join forces to provide innovative pharmacovigilance (PV) safety services for treatments targeting rare autoimmune disorders. This collaboration leverages technology to enhance patient safety and support the development of new treatments for these complex conditions.
- LabCorp Expands Diagnostics Reach (Oct 2023): LabCorp strengthens its presence in the diagnostics market by acquiring outreach laboratory services and assets from Baystate Health. This acquisition broadens LabCorp's reach across Massachusetts, potentially improving access to critical laboratory services for patients in the region.
 Report Scope
Report Scope
Report Attribute |
Details |
Market Size in 2023 |
USD 68.82 Billion |
Projected Market Size in 2032 |
USD 134.65 Billion |
CAGR Growth Rate |
7.74% CAGR |
Base Year |
2023 |
Forecast Years |
2024-2032 |
Key Market Players |
Axcent Advanced Analytics, BIO Agile Therapeutics, Charles River Laboratories, Dove Quality Solutions, Eurofins Scientific, Firma Clinical Research, Fortrea Inc., Frontage Holding Corporation, Geneticist In., ICON Plc, IQVIA Inc., Laboratory Corporation of America Holdings, Medpace, Novotech Health Holding, Parexel International, Pharmaron, Syneos Health, Thermo Fisher Scientific Inc., WuXi AppTec, and others., and Others. |
Key Segment |
By Service Type, By Application, By End-User, and By Region |
Major Regions Covered |
North America, Europe, Asia Pacific, Latin America, and the Middle East &, Africa |
Purchase Options |
Request customized purchase options to meet your research needs. Explore purchase options |
 Contract Research Organization (CRO) Services Market: Regional Analysis
Contract Research Organization (CRO) Services Market: Regional Analysis
The U.S. contract research organization (CRO) services market is expected to grow at a CAGR of 7.92% between 2024 to 2032.
North America, particularly the United States, is a dominant player in the global CRO market. North America has captured a market share of around 33.86% in 2023. This region is characterized by a robust pharmaceutical sector, substantial investments in biotechnology and medical research, and a strong regulatory framework governed by the FDA. The presence of leading pharmaceutical companies and advanced healthcare infrastructure supports a thriving CRO industry. The market here is mature, with a high demand for outsourcing clinical trials and regulatory compliance services.
Europe also holds a significant share of the CRO market, driven by its advanced healthcare systems, strong focus on research and development, and supportive government policies. The European Medicines Agency (EMA) plays a crucial role in shaping the regulatory landscape, which influences the operations of CROs. Countries like Germany, France, and the UK are notable contributors to the market growth in this region.
The Asia-Pacific region is the fastest-growing market for CRO services, driven by lower operational costs, a rapidly expanding healthcare sector, and increasing governmental support for pharmaceutical research in countries like China and India. The region is becoming a preferred destination for clinical trials due to its diverse patient population and the improving regulatory environment. The growth in this region is further accelerated by rising investments in healthcare infrastructure and a growing focus on chronic diseases.
Latin America presents growth opportunities for CROs due to its regulatory reforms, skilled clinical workforce, and diverse genetic pool that is advantageous for clinical trials. Countries like Brazil and Mexico are leading this trend, with an increasing number of pharmaceutical companies looking to leverage the region’s potential in clinical research.
While still emerging, the Middle East and Africa region is experiencing growth in the CRO market due to increasing investments in healthcare systems, a rising burden of diseases, and an improving regulatory framework. The region offers untapped opportunities due to its large, diverse population and increasing healthcare expenditure.
 Contract Research Organization (CRO) Services Market: List of Key Players
Contract Research Organization (CRO) Services Market: List of Key Players
Some of the main competitors dominating the global contract research organization (CRO) services market include;
- Axcent Advanced Analytics
- BIO Agile Therapeutics
- Charles River Laboratories
- Dove Quality Solutions
- Eurofins Scientific
- Firma Clinical Research
- Fortrea, Inc.
- Frontage Holding Corporation
- Geneticist In.
- ICON Plc
- IQVIA Inc.
- Laboratory Corporation of America Holdings
- Medpace
- Novotech Health Holding
- Parexel International
- Pharmaron
- Syneos Health
- Thermo Fisher Scientific Inc.
- WuXi AppTec
The global contract research organization (CRO) services market is segmented as follows:
 By Service Type Segment Analysis
By Service Type Segment Analysis
- Clinical Trial Services
- Preclinical Services
- Regulatory Services
- Laboratory Services
- Others
 By Application Segment Analysis
By Application Segment Analysis
- Oncology
- Central Nervous System (CNS) Disorder
- Cardiovascular Diseases
- Infectious Disease
- Others
 By End-User Segment Analysis
By End-User Segment Analysis
- Pharmaceutical And Biotechnology Companies
- Medical Device Companies
- Academic Institutes
 By Regional Segment Analysis
By Regional Segment Analysis
- North America
- The U.S.
- Canada
- Mexico
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- Southeast Asia
- Rest of Asia Pacific
- The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
Industry Major Market Players
- Axcent Advanced Analytics
- BIO Agile Therapeutics
- Charles River Laboratories
- Dove Quality Solutions
- Eurofins Scientific
- Firma Clinical Research
- Fortrea, Inc.
- Frontage Holding Corporation
- Geneticist In.
- ICON Plc
- IQVIA Inc.
- Laboratory Corporation of America Holdings
- Medpace
- Novotech Health Holding
- Parexel International
- Pharmaron
- Syneos Health
- Thermo Fisher Scientific Inc.
- WuXi AppTec

Copyright © 2024 - 2025, All Rights Reserved, Facts and Factors


