Search Market Research Report
Rare Diseases Treatment Market Size, Share Global Analysis Report, 2025 – 2034
Rare Diseases Treatment Market Size, Share, Growth Analysis Report By Drug Type (Biologics, Biosimilar, Small Molecules), By Therapeutic Area (Cancer, Neurological Conditions, Cardiovascular Conditions, Musculoskeletal Conditions, Hematologic Disorders, Infectious Diseases, Metabolic Disorders, Endocrine Disorders, and Others), By Route of Administration (Oral, Injectable, and Others), By Distribution Channel (Hospital Pharmacy, Specialty Pharmacy, Online Pharmacy), And By Region - Global Industry Insights, Overview, Comprehensive Analysis, Trends, Statistical Research, Market Intelligence, Historical Data and Forecast 2025 – 2034
Industry Insights
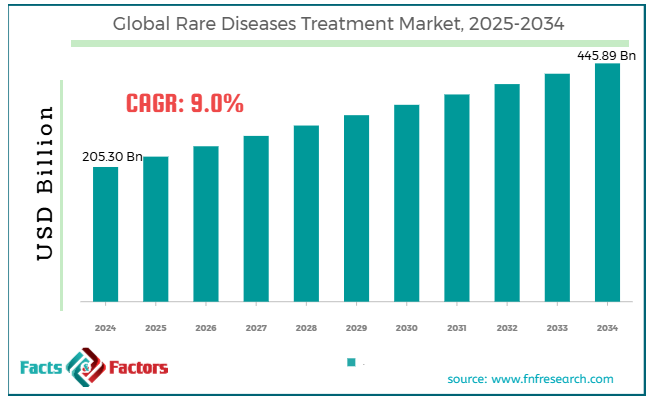
[221+ Pages Report] According to Facts & Factors, the global rare diseases treatment market size was worth around USD 205.30 billion in 2024 and is predicted to grow to around USD 445.89 billion by 2034, with a compound annual growth rate (CAGR) of roughly 9% between 2025 and 2034.
 Market Overview
Market Overview
Rare diseases are illnesses or medical conditions that affect a smaller portion of the population, mostly less than 1 in 2000 individuals. These diseases may not be easily treated due to restricted knowledge, lack of research funding, and a small patient population.
Since rare diseases are uncommon, they usually demand research-focused approaches and specialized care to develop cures or novel treatments. The global rare diseases treatment market will progress substantially owing to the improvements in gene therapy and biotechnology, growing investment in research, and increasing patient awareness.
The growth of genetic engineering and gene therapy is playing a significant role in treating rare diseases. Genetic treatments may aim at the origin of the medical condition, providing the likelihood of effectively managing or curing the illness. Modern techniques like RNA-based therapies, CRISPR, and gene editing can potentially transform the treatment of rare diseases.
Moreover, the private and public sectors heavily invest in research to develop and identify rare disease treatments. Also, increasing awareness among healthcare providers and the public supports the identification of rare diseases more effectively. Hence, improved diagnostic rates and awareness fuel the demand for such illnesses since more individuals are diagnosed with rare diseases.
Nonetheless, the industry is projected to be hampered by the high cost of treatment and the lack of awareness of the disease. Treatment for rare illnesses needs costly therapies and medications, which may increase financial pressure on healthcare systems and patients. The costly development of these drugs is among the leading restraining factors, restricting the amount of available treatments.
Furthermore, due to a lack of awareness among medical professionals, rare diseases are mostly undiagnosed or misdiagnosed. Delays may hamper early treatment, limiting medication demand and impacting patient results.
Yet, some opportunistic factors include the development of orphan drugs and the innovation of cell and gene therapy. Regulatory bodies and governments are offering research grants, incentives, and more to encourage the emergence of novel treatments.
In addition, cell-based and gene therapies hold immense promise for managing and curing such illnesses. The present improvements in these domains offer companies fresh ways to cure these rare diseases.
 Key Insights:
Key Insights:
- As per the analysis shared by our research analyst, the global rare diseases treatment market is estimated to grow annually at a CAGR of around 9% over the forecast period (2025-2034)
- In terms of revenue, the global rare diseases treatment market size was valued at around USD 205.30 billion in 2024 and is projected to reach USD 445.89 billion by 2034.
- The rare diseases treatment market is projected to grow significantly due to the increasing awareness of rare diseases, the surging healthcare investment for orphan drugs, and the increasing patient pool.
- Based on drug type, the biologics segment is expected to lead the market, while the small molecules segment is expected to register considerable growth.
- Based on the therapeutic area, the cancer segment is the dominating segment among others, while the musculoskeletal conditions segment is projected to witness sizeable revenue over the forecast period.
- Based on the route of administration, the injectable segment held the maximum portion of the market, while the oral segment will gain a notable market share over the forecast period.
- Based on the distribution channel, the specialty pharmacy segment is expected to lead the market compared to the hospital pharmacy segment.
- Based on region, North America is projected to dominate the global market during the estimated period, followed by Europe.
 Growth Drivers
Growth Drivers
- Developments in gene therapy and biotechnology fuel the rare diseases treatment market growth
Gene therapy is an innovative development in biotechnology that aims to address the fundamental genetic causes of rare diseases. Unlike the earlier treatment, which only managed symptoms, gene therapy effectively replaces and corrects faulty genes, thus offering a potential treatment for rare illnesses.
A gene therapy for Spinal Muscular Atrophy (SMA) was developed by Novartis, named Zolgensma, in 2024. It became the costliest drug on a global scale, rated at USD 2.1 million per dosage. This denotes the rising emphasis on gene therapies for curing rare disorders, thus driving the rare disease treatment industry.
In February 2025, Mirum Pharmaceuticals' Ctexli gained approval from the U.S. FDA for treating cerebrotendinous xanthomatosis, a rare illness (genetic). This authorization highlights the rising role of gene therapies that were earlier incurable.
- Growth of the orphan drug market to propel the industry growth
The global orphan drug market is speedily expanding owing to the incentives that boost pharma companies to introduce treatments for rare illnesses. While orphan drugs are costly, they offer pharmaceutical firms uniqueness in the market, increasing their profitability despite a small population of patients.
Sanofi announced its merger with Provention Bio in March 2025. The company focuses on autoimmune illnesses, a rare group comprising medical conditions like type 1 diabetes. This merger expands the orphan drug portfolio of Sanofi, thus highlighting the rising emphasis on treating rare illnesses.
 Restraints
Restraints
- Regulatory barriers and complexities unfavorably affect the progress of the rare diseases treatment market
While incentives like the Orphan Drugs Act are available in the United States and similar laws globally, the regulatory scenario for treating rare diseases is still complicated and may create significant delays in launching treatments to the market. Also, varying policies across diverse nations may further complicate the process.
‘Cytokinetics' witnessed a delay in acceptance of its novel drug for rare cardiac disease in 2024 owing to extra clinical experiments needed by the EMA (European Medicines Agency). This is commonly seen in the said market, where unreliable regional regulatory processes add cost and time to the development.
The inconsistency and complexity of the regulatory process in diverse regions may slow the launch of different novel therapies to the industry, thus delaying access for needy patients.
 Opportunities
Opportunities
- The growing use of AI in drug discovery is a key opportunity in the rare diseases treatment market
Artificial intelligence is actively used to boost the drug discovery processes, especially for rare diseases where conventional techniques are usually costly and slow. AI can effectively analyze large quantities of clinical, genetic, and chemical data to recognize impending drug candidates efficiently and faster.
AI's capability to renovate drug development by decreasing the cost and time of research denotes a significant opportunity for treating rare illnesses. This brings novel therapies to the market more effectively and quickly.
BenevolentAI declared a collaboration with Sanofi in 2025 to use artificial intelligence to recognize new drug targets for rare neurological illnesses. It aimed to simplify the discovery of novel treatments and lessen the time to market for life-preserving treatments.
 Challenges
Challenges
- Accessibility and ethical concerns to limit the growth of the rare diseases treatment market
Ethical questions for equitable access and affordability of treatments are major. Most rare illness therapies are expensive for low-income patients, and this expense may restrict access even for high-income individuals. In addition, the problem of treating rare diseases in budget-constrained nations and clinical trial access further creates differences in worldwide healthcare.
As per the WHO, in the middle-income and low-income nations, nearly 90% of patients with rare diseases lack access to suitable treatments owing to the healthcare system and economic constraints.
 Report Scope
Report Scope
Report Attribute |
Details |
Market Size in 2024 |
USD 205.30 Billion |
Projected Market Size in 2034 |
USD 445.89 Billion |
CAGR Growth Rate |
9.0% CAGR |
Base Year |
2024 |
Forecast Years |
2025-2032 |
Key Market Players |
Sanofi, Novartis, Roche, Pfizer, Alexion Pharmaceuticals, Shire Pharmaceuticals, Bayer, Biogen, Amgen, Bristol-Myers Squibb, AbbVie, Vertex Pharmaceuticals, Eli Lilly and Company, Regeneron Pharmaceuticals, Gilead Sciences., and others. |
Key Segment |
By Drug Type, By Therapeutic Area, By Route of Administration, By Distribution Channel, and Region |
Major Regions Covered |
North America, Europe, Asia Pacific, Latin America, and the Middle East &, Africa |
Purchase Options |
Request customized purchase options to meet your research needs. Explore purchase options |
 Segmentation Analysis
Segmentation Analysis
The global rare diseases treatment market is segmented based on product type, material type, end-user industry, and region.
Based on drug type, the global rare diseases treatment industry is divided into biologics, biosimilar, and small molecules. The biologics segment is the leading segment, followed by the tiny molecules segment. Biologic drugs are complex and large molecules, mainly obtained from living organisms that treat genetic and rare diseases. These drugs have showcased major promises in treating conditions that do not have therapeutic options, especially for autoimmune, genetic, and blood conditions. Some of the biologics utilized for rare illnesses comprise Eculizumab (Soliris) for PNH and aHUS conditions and Factor IX and VIII therapies for hemophilia B and A. Mepolizumab (Nucala) is also used to treat rare autoimmune illnesses.
Based on the route of administration, the global rare diseases treatment industry is segmented as oral, injectable, and others. The injectable segment held a considerable share of the market compared to others. Several biologic treatments broadly used to treat rare illnesses need injection owing to their large molecular size. This prevents them from effective absorption via the digestive tract. Injectables are also opted for treatments that require direct delivery in the tissues or bloodstream for immediate action. The industry is projected to dominate, emphasizing gene therapies and injectable biologics for genetic and rare illnesses.
Based on distribution channel, the global market is segmented as hospital pharmacy, specialty pharmacy, and online pharmacy. The specialty pharmacies are the leading distribution channel in the market since they emphasize distributing medications for chronic, complex, and rare illnesses that frequently need special handling, monitoring, and storage. These pharmacies play a vital role in handling the distribution of costly orphan drugs and biologics that are commonly used in treating rare disorders.
 Regional Analysis
Regional Analysis
- North America to witness significant growth over the forecast period
North America held a maximum share of the global rare diseases treatment market in 2024 owing to strong healthcare infrastructure, high spending in healthcare, and rising patient awareness. North America, mainly the US, has a well-developed healthcare infrastructure, enabling speedy development, the distribution of therapies, and approval for rate illnesses.
The U.S. FDA's Orphan Drug Act offers incentives for developing treatments for rare diseases, thus fueling innovation. North America holds high healthcare expenses, allowing access to costly treatments.
Furthermore, the region has a high level of advocacy groups and patient awareness that pressurize access to government support and treatment for research. Groups like NORD advocate for access to rare disease treatment.
Europe will hold a considerable share of the global rare diseases treatment market over the estimated period owing to strong regulatory policies, high investment, and collaborative R&D. Europe has a well-developed regulatory system through EMA that supports the approval and development of orphan drugs. This stimulates the growth of the rare disease treatment market.
The region also holds high healthcare spending, mainly in France, Germany, and the United Kingdom, allowing access to the treatment of rare illnesses. Germany alone invested nearly $ 400 billion in healthcare, supporting the availability of rare illnesses therapies.
Furthermore, the region has several innovative biotech companies and research collaborations emphasizing rare illnesses. The European Union backs these efforts via funding plans like Horizon 2020, which contribute to research on orphan drugs and rare illnesses.
 Competitive Analysis
Competitive Analysis
The global rare diseases treatment market is led by players like:
- Sanofi
- Novartis
- Roche
- Pfizer
- Alexion Pharmaceuticals
- Shire Pharmaceuticals
- Bayer
- Biogen
- Amgen
- Bristol-Myers Squibb
- AbbVie
- Vertex Pharmaceuticals
- Eli Lilly and Company
- Regeneron Pharmaceuticals
- Gilead Sciences
 Key Market Trends
Key Market Trends
- Growing emphasis on cell and gene therapies:
Cell and gene therapies are growing as transformative treatments for various genetic illnesses, offering the potential for one-time treatments. Modernizations in viral vector-based therapies and CRISPR gene editing result in novel treatments for earlier incurable rare illnesses.
- Growing investment in orphan drugs:
Rising investment in orphan drugs is fueled by both biotech startups and pharmaceutical companies. Government initiatives like extended market exclusivity and tax credits are boosting the growth of different orphan drugs for rare illnesses.
The global rare diseases treatment market is segmented as follows:
 By Drug Typ Segment Analysis
By Drug Typ Segment Analysis
- Biologics
- Biosimilar
- Small Molecules
 By Therapeutic Area Segment Analysis
By Therapeutic Area Segment Analysis
- Cancer
- Neurological Conditions
- Cardiovascular Conditions
- Musculoskeletal Conditions
- Hematologic Disorders
- Infectious Diseases
- Metabolic Disorders
- Endocrine Disorders
- Others
 By Route of Administration Segment Analysis
By Route of Administration Segment Analysis
- Oral
- Injectable
- Others
 By Distribution ChannelSegment Analysis
By Distribution ChannelSegment Analysis
- Hospital Pharmacy
- Specialty Pharmacy
- Online Pharmacy
 By Regional Segment Analysis
By Regional Segment Analysis
- North America
- The U.S.
- Canada
- Mexico
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- Southeast Asia
- Rest of Asia Pacific
- The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
Industry Major Market Players
- Sanofi
- Novartis
- Roche
- Pfizer
- Alexion Pharmaceuticals
- Shire Pharmaceuticals
- Bayer
- Biogen
- Amgen
- Bristol-Myers Squibb
- AbbVie
- Vertex Pharmaceuticals
- Eli Lilly and Company
- Regeneron Pharmaceuticals
- Gilead Sciences
Frequently Asked Questions

Copyright © 2024 - 2025, All Rights Reserved, Facts and Factors


