Search Market Research Report
Viral Vectors And Plasmid DNA Manufacturing Market Size, Share Global Analysis Report, ors And Plasmid DNA Manufacturing Market By Product Type (Viral Vectors, Plasma DNA, and Non-Viral DNA Vectors), By Application (Cancer, Inherited Disorder, Infectious Diseases, and Others), and By End-User (DNA Vaccines, Gene Therapy, Immunotherapy, and Others): Global Industry Perspective, Comprehensive Analysis, and Forecast, 2021 – 2026
Viral Vectors And Plasmid DNA Manufacturing Market By Product Type (Viral Vectors, Plasma DNA, and Non-Viral DNA Vectors), By Application (Cancer, Inherited Disorder, Infectious Diseases, and Others), and By End-User (DNA Vaccines, Gene Therapy, Immunotherapy, and Others): Global Industry Perspective, Comprehensive Analysis, and Forecast, 2021 – 2026
Industry Insights
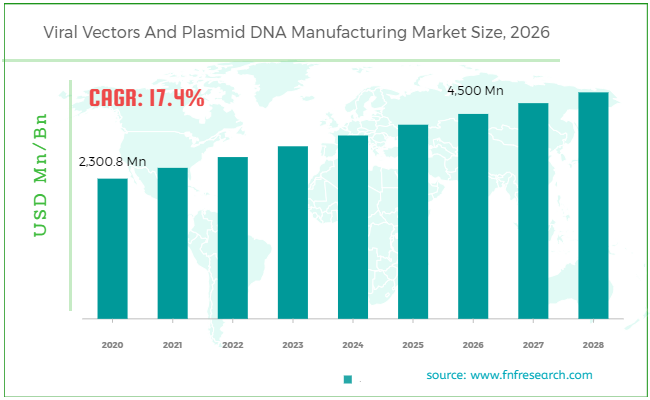
[196+ Pages Report] According to a Facts & Factors report, the global viral vectors and plasmid DNA manufacturing market was estimated at approximately USD 2,300.8 Million in 2020 and is projected to produce revenue worth approximately USD 4,500 Million by the end of 2026, growing at a CAGR of approximately 17.4% between 2021 and 2026.
 Global Viral Vectors & Plasmid DNA Manufacturing Market: Overview
Global Viral Vectors & Plasmid DNA Manufacturing Market: Overview
A viral vector is a molecular biologist's instrument for transporting genetic material into cells. Viruses have evolved specific ways to transport their DNA into the cells they infect. Retrovirus, lentivirus, adenovirus, herpes simplex virus, and adeno-adenovirus are all examples of viral vectors that may be employed to transfer nucleic acids into the genetic composition of cells. The plasma DNA is a lump of DNA materials gained from different tissues and cells, and it is a microbial circular, smaller, and extrachromosomal DNA duplicate self-reliantly utilized in recombinant DNA technology and genetic engineering. The plasma DNA is categorized into GMP Grade Plasmid DNA, Non-GMP grade plasmid DNA, and high-quality plasmid DNA. The high-quality plasmid DNA is utilized in the GMP-compliant making of recombinant viruses, RNA, antibodies.

 Global Viral Vectors & Plasmid DNA Manufacturing Market: Growth Factor
Global Viral Vectors & Plasmid DNA Manufacturing Market: Growth Factor
The viral vectors and plasmid DNA manufacturing market size is expected to enlarge at a significant growth rate during the forecast period. The increasing demand for viral vector-based vaccines for infectious diseases and the constant change in the treatment model of numerous life-threatening diseases using advanced therapies are some key factors that are driving the growth of the market across the globe. The rising employment of viral vectors and plasmid DNA for the research and development of highly developed therapies is likely to fuel the global viral vectors and plasmid DNA manufacturing market. Furthermore, the competition in the arena is projected to strengthen over the analysis period due to the resultant boost in manufacturing potential and escalating gene therapy approvals.
However, the major factors restraining the growth of the market are ethical, regulatory, and scientific challenges regarding gene therapy, the manufacturing capacity restrictions, and production challenges concerning to large-scale manufacturing of vectors. Conversely, the surge in healthcare infrastructure will generate several opportunities in the sector, where the demand for viral vectors and plasmid DNA will boost across the world. Also, the COVID-19 pandemic is offering lucrative opportunities for the top players in the market.
 Global Viral Vectors & Plasmid DNA Manufacturing Market: Segmentation
Global Viral Vectors & Plasmid DNA Manufacturing Market: Segmentation
The global viral vectors & plasmid DNA manufacturing market has been classified into product type, application, and end-user. The global market of viral vectors & plasmid DNA manufacturing based on the product type has been segmented into viral vectors, plasma DNA, and non-viral DNA vectors. Based on the application, the market has been divided into cancer, inherited disorder, infectious diseases, and others. In terms of the end-user, the market has been separated into DNA vaccines, gene therapy, immunotherapy, and others.
 Report Scope
Report Scope
Report Attributes |
Details |
Market Size in 2020 |
USD 2,300.8 Million |
Projected Market Size in 2026 |
USD 4,500 Million |
CAGR Growth Rate |
17.4% CAGR |
Base Year |
2020 |
Forecast Years |
2021 - 2026 |
Key Market Players |
Merck KGaA, FUJIFILM Diosynth Biotechnologies, Lonza, Thermo Fisher Scientific, Catalent Inc., Cobra Biologics, Wuxi Biologics, WaismanBiomanufacturing, Takara Bio Inc., Genezen laboratories, MiltenyiBiotec GmbH, Batavia Biosciences, SIRION Biotech GmbH, BioNTech IMFS GmbH, Virovek Incorporation, Audentes Therapeutics, RegenxBio Inc., BioMarin Pharmaceutical, YPOSKESI, and Advanced BioScience |
Key Segment |
By Product Type, By Application, By End-User, and By Region |
Major Regions Covered |
North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
Purchase Options |
Request customized purchase options to meet your research needs. Explore purchase options |
 Global Viral Vectors & Plasmid DNA Manufacturing Market: Regional Analysis
Global Viral Vectors & Plasmid DNA Manufacturing Market: Regional Analysis
North America is leading the global market of viral vectors and plasmid DNA manufacturing market and is contributing to the largest CAGR. The factors contributing to the market growth in this region are the occurrence of the numerous institutes and centers engaged in the research and development of superior therapies. As a result of the growing regulatory approvals for superior therapies, the United States contributed extensively to regional income production. Also, companies based in the United States are investing a major amount to develop their production potential.
 Global Viral Vectors & Plasmid DNA Manufacturing Market: Competitive Players
Global Viral Vectors & Plasmid DNA Manufacturing Market: Competitive Players
- Merck KGaA
- FUJIFILM Diosynth Biotechnologies
- Lonza
- Thermo Fisher Scientific
- Catalent Inc.
- Cobra Biologics
- Wuxi Biologics
- WaismanBiomanufacturing
- Takara Bio Inc.
- Genezen laboratories
- MiltenyiBiotec GmbH
- Batavia Biosciences
- SIRION Biotech GmbH
- BioNTech IMFS GmbH
- Virovek Incorporation
- Audentes Therapeutics
- RegenxBio Inc.
- BioMarin Pharmaceutical
- YPOSKESI
- Advanced BioScience
are some market companies operating in the market of vectors & plasmid DNA manufacturing worldwide. Furthermore, these market players are working in partnership with major operating stakeholders as well as regulatory bodies, non-industry, and industry players, and others to deal with the challenges efficiently in viral vector production.
 Global Viral Vectors & Plasmid DNA Manufacturing Market: By Product Type
Global Viral Vectors & Plasmid DNA Manufacturing Market: By Product Type
- Viral Vectors
- Plasma DNA
- Non-Viral DNA Vectors
 Global Viral Vectors & Plasmid DNA Manufacturing Market: By Application
Global Viral Vectors & Plasmid DNA Manufacturing Market: By Application
- Cancer
- Inherited Disorder
- Infectious Diseases
- Others
 Global Viral Vectors & Plasmid DNA Manufacturing Market: By End-User
Global Viral Vectors & Plasmid DNA Manufacturing Market: By End-User
- DNA Vaccines
- Gene Therapy
- Immunotherapy
- Others
 Global Viral Vectors & Plasmid DNA Manufacturing Market: Regional Segmentation Analysis
Global Viral Vectors & Plasmid DNA Manufacturing Market: Regional Segmentation Analysis
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Industry Major Market Players
- Merck KGaA
- FUJIFILM Diosynth Biotechnologies
- Lonza
- Thermo Fisher Scientific
- Catalent Inc.
- Cobra Biologics
- Wuxi Biologics
- WaismanBiomanufacturing
- Takara Bio Inc.
- Genezen laboratories
- MiltenyiBiotec GmbH
- Batavia Biosciences
- SIRION Biotech GmbH
- BioNTech IMFS GmbH
- Virovek Incorporation
- Audentes Therapeutics
- RegenxBio Inc.
- BioMarin Pharmaceutical
- YPOSKESI
- Advanced BioScience
Frequently Asked Questions

Copyright © 2024 - 2025, All Rights Reserved, Facts and Factors


